Recent FDA Approvals for Drug-eluting Balloons
In November 2019, the FDA cleared the Medtronic In.Pact AV DCB for the treatment of failing AV access in patients with end-stage renal disease (ESRD) undergoing dialysis.
Over time, vessel restenosis limits the ability to use AV fistulae effectively. In order to restore function, patients often undergo one to three maintenance procedures per year, which can result in significant disruptions to critical hemodialysis care and increased costs to the healthcare system. Pivotal randomized trial results from the IN.PACT AV Access Trial have shown the In.Pact AV paclitaxel-coated balloon can extend the time between reinterventions by maintaining AV access site patency, therefore maximizing a patient’s uninterrupted access to lifesaving dialysis care.
“In many cases, AV fistula are considered lifelines for patients with ESRD as they are the primary access point for life-saving dialysis treatment. When these access sites fail, patients experience delays in their dialysis treatment and require multiple reinterventions to keep the site functioning,” said Vincent Gallo, M.D., interventional radiologist at Holy Name Medical Center in Teaneck, New Jersey, and an investigator for the IN.PACT AV Access Trial. “With this approval physicians now have access to a safe and extremely effective therapy to slow the progression of restenosis, which results in fewer reinterventions and disruptions in care for these patients.”
Philips Healthcare gained FDA in October 2019 for two new Stellarex 0.035-inch low-dose DCB portfolio. The new 200 and 150 mm Stellarex balloons are indicated for treatment of de novo and restenotic lesions in native superficial femoral or popliteal arteries.
All Philips’ Stellarex DCBs feature EnduraCoat technology, a coating consisting of a polyethylene glycol excipient with amorphous and crystalline paclitaxel particles dispersed in it. The coating provides efficient drug transfer and effective drug residency coupled with high coating durability and minimal particulate loss, thereby enabling a low therapeutic drug dose. The Stellarex balloon is now available in 40, 60, 80, 100, 120, 150 and 200 mm lengths for the treatment of lesions in the superficial femoral and popliteal arteries with vessel diameters of 4-6 mm.
In June 2018, the FDA cleared 200 and 250 mm lengths of the Medtronic In.Pact Admiral DCB to treat long superficial femoral artery (SFA) lesions in patients with PAD.
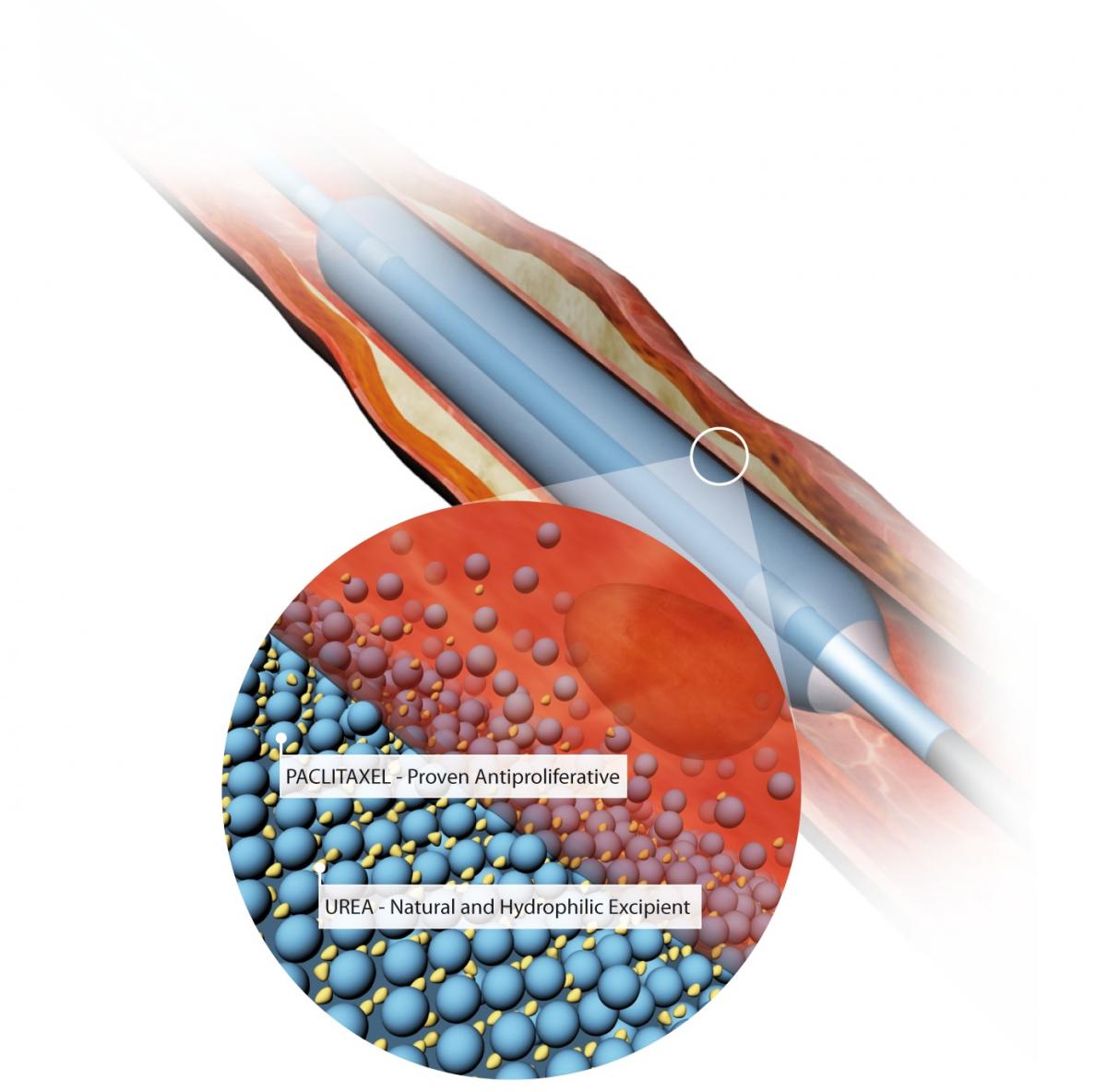
The Medtronic In.Pact Admiral drug-coated balloon, with an illustration showing the balloon’s urea excipient drug carrier and the paclitaxel drug.
Article Source: https://www.dicardiology.com/article/recent-developments-drug-coated-balloons